Vinews
No. 8 — June 22, 2020
Contents:
- As Temperatures Rise and the Pest Complex Increases, Be Aware of Tank Mixes
- Some Grape Virus Combinations Appear to Be More Detrimental to Grapevines
- Japanese Beetles Update
- Cumulative Growing Degree Days (Base 50) for the Seven Grape Growing Regions of Missouri from April 1 to Sept. 5, 2020
- Weather Outlook
As Temperatures Rise and the Pest Complex Increases, Be Aware of Tank Mixes
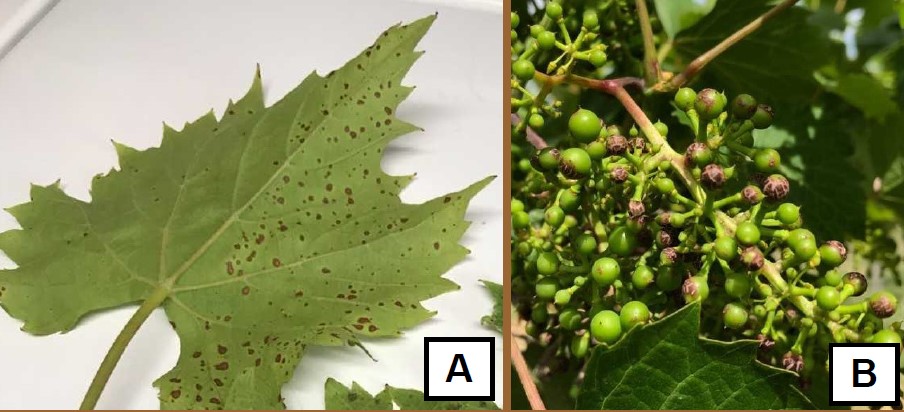
You need to consider several factors prior to making a complex spray tank mixture. With Japanese beetles becoming more prevalent and fungal issues still a concern, there is often consideration of tank mixing multiple pesticides. Tank mixing has several advantages; single application, delivering more than one mode of action directed at a pest and tank mixing saves time. If you have tank mixed certain pesticides before and were satisfied with the results, then there is little concern for phytotoxicity. However, if you are considering a new tank mix, you should initially try the mixture on a few vines at least few days prior to a full-blown vineyard application. Symptomology as a result of phytotoxicity often appears relatively fast compared to symptomology as a result of a pathogen infection.
There are classic differences between disease symptomology and phytotoxicity. Disease symptoms appear random on leaf tissue or berries. Whereas phytotoxicity often appears on only the tissue that was exposed to the spray droplets (Figure 1). Fruit clusters typically only have phytotoxicity symptoms on the side of the cluster that was directly exposed to the spray application. Similarly, leaves on the outside of the canopy will have phytotoxicity symptoms whereas leaves within the canopy will not have or will have reduced symptoms.
As we enter the summer months consider the air temperature prior to making a pesticide application. Try to avoid making any pesticide application when air temperatures are above 85 degrees F. Some fungicides such as sulfur and Captan can become phytotoxic at high air temperatures.
Pay attention to the Captan label. Do not spray Captan products immediately before or after a short period when an oil has been applied. Remember emulsifiable concentrates or EC formulations often contain a surfactant which when mixed with Captan, the resulting mixture may cause phytotoxicity. Captan only becomes phytotoxic if it is delivered into the cells of green tissue. Both oils and EC formulated pesticides can help deliver Captan to unintended tissue such as grape tissue.
In summary, if you are applying a tank mix of pesticide products that you have not applied before, it would be worth the time to make a small batch mixture and try out on a few grapevines to determine the safety of the tank mixture. Pay attention to the air temperatures prior to a pesticide application, try to avoid pesticide applications when the air temperatures are above 85 degrees F.
Some Grape Virus Combinations Appear to Be More Detrimental to Grapevines


Some very preliminary observations in the field suggest that certain virus combinations are more damaging than others (Figure 2). Over the past year, Jim Schoelz and I have been identifying grapevines that have certain virus combinations. In addition, we also are identifying grapevines that have only one of the viruses found in the combination. We also are identifying grapevines that have no virus at all. As an example we identify grapevines that have Grapevine Red Blotch Virus (GRBV) + Grapevine Vein Clearing Virus (GVCV), grapevines that have only GRBV and grapevines that have only GVCV, and grapevines that have no virus at all.
This week we observed some of these grapevines in commercial vineyards in which we are researching. Preliminary observations suggest that GRBV+GVCV has a detrimental impact on grapevine growth and development compared to grapevines with either GRBV or GVCV alone. Realize these are preliminary observations and our research is just at its infancy. To further define which virus combinations are the most detrimental to grapevines, we are developing an experimental planting of grapevine cultivars with different virus combinations. The idea is to quantify grapevine growth and development in these grapevines and to determine which viruses and virus combinations are the most detrimental to Missouri grapevine cultivars.
There is a great need to determine which grapevine viruses are having a negative impact on Missouri grape cultivars. Grapevine red blotch virus was found in 35 percent of the samples in a statewide survey of vineyards in Missouri in 2007. Unlike Vitis vinifera cultivars, the American Heritage Cultivars grown in Missouri such as Vignoles, Vidal blanc, Valvin muscat, Norton and Chambourcin do not display symptomology when infected with GRBV. At this time we do not know if GRBV is having a negative impact on grapevine growth and development, berry quality or grapevine longevity. Current theory of Vitis vinifera suggests that the development of red blotches on the leaves decreases the photosynthetic area resulting in reduced brix and or stalled out sugar accumulation. Since Missouri grape cultivars do not develop classic red blotch symptomology when infected with GRBV, the question becomes what impact is GRBV having?
In the future, we hope to answer that question as well as what virus combinations are the most detrimental to grapevine growth and development and berry quality. Similar to grapevines infected with GRBV, grapevines (Vidal blanc and St. Vincent) infected with Grapevine Leafroll associated Virus-3 (GLRaV-3) do not display classic leafroll symptomology. Initially grapevines infected with GLRaV-3 were identified in the vineyard using ELISA. To further determine if the vines identified in the vineyard had GLRaV-3 graft indexing was performed. Graft indexing involves grafting a scion infected with GLRaV-3 onto a Vitis vinifera cultivar that is highly sensitive to leafroll disease. The GLRaV-3 will travel from the grafted scion to the rootstock and the developing shoots from the rootstock display Grapevine leafroll disease symptomology. This research was completed by Laszlo Kovacs et al. in 2001 at Missouri State University.
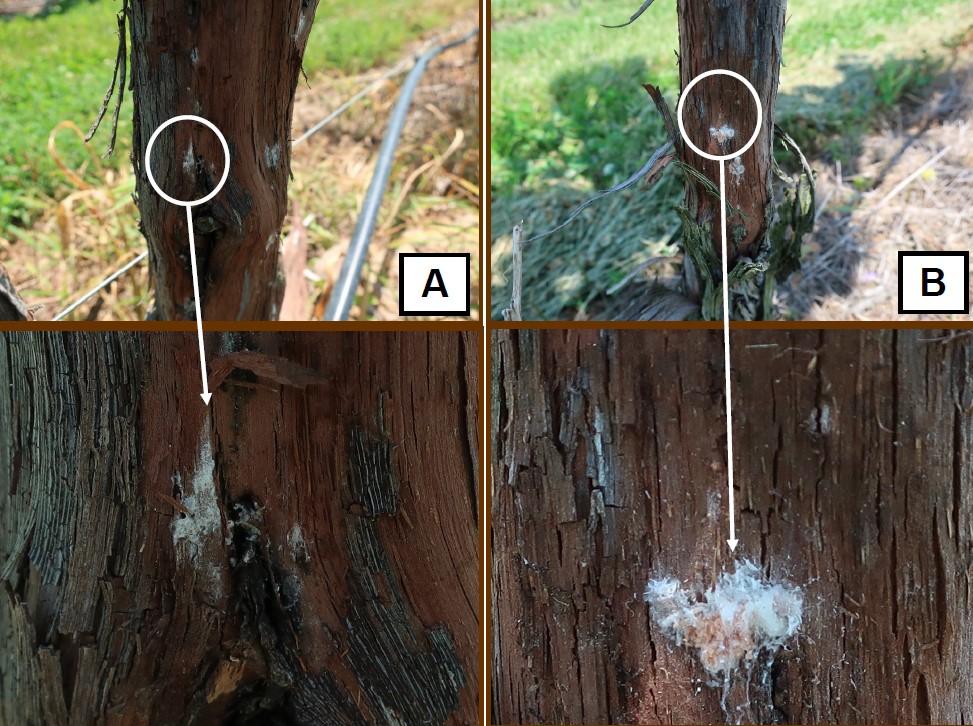
I bring this last example to your attention because this week I again had the opportunity to evaluate a couple of vineyard blocks for mealybugs. Mealybugs are the vector of GLRaV-3 as well as some other strains of grapevine leafroll. In each block evaluated, adult mealybugs as well as egg clutches were very prevalent (Figure 3 and 4). The management of leafroll disease is managing the insect vectors that spread the GLRaV-3. So take some time and strip some bark and start evaluating your vineyard blocks for mealybugs. Next month the eggs will hatch producing the second generation of mealybugs that are clearly visible with the naked eye.
Japanese Beetles Update

Update for week of June 22, 2020
Pheromone trap captures of Japanese beetles were over 5,000 for southeast and southwest Missouri this week. In vineyards in central Missouri and East Central Missouri this week, I observed very few Japanese beetles and very little to no damage from Japanese beetles. Be alert as Japanese beetles are emerging.
Update for week of June 15, 2020
This week I observed no Japanese beetles in 6 different vineyard blocks I scouted in the New Haven and Hermann area on Thursday, June 18. Today, June 19, I observed Japanese beetles in Mexico, MO. With rain predicted the next few days, adults will have an easier time emerging from the soil. Scout every day if possible. Research has shown that Japanese beetles are not attracted to other Japanese beetles. In other words Japanese beetles do not have congregation pheromones. In grapes it has been shown that Japanese beetles are attracted to grapevines that have been fed on by Japanese beetles. The injured grapevines put out signals that attract more Japanese beetles. The initial influx of beetles is typically always on border rows and so scout border rows first, especially border rows along wooded areas and grassy strips.
First Report of Japanese Beetles for the 2020 Season
June 5, 2020, Japanese beetles were reported in Central Northeast Missouri, specifically Boone County. The Japanese beetles were reported on the University of Missouri Integrated Pest Management website. The trapping network has eleven sites around Missouri trapping for Japanese beetles. The Japanese beetles were caught in a pheromone trap. On the IPM website you can sign up to receive pest alerts. The trapping network is conducted by MU regional agronomy and horticulture extension professionals.
With wet soil conditions throughout most of Missouri there will be an opportunity for the adults to easily emerge in the coming days and weeks. A natural enemy of Japanese beetles has been established in Missouri. A small wasp — Tiphia vernalis (Hymenoptera Tiphiidae) — that parasitizes the Japanese Beetle larva. Hopefully biocontrol agents will help reduce Japanese beetle populations.
Cumulative Growing Degree Days (Base 50) for the Seven Grape Growing Regions of Missouri from April 1 to June 20, 2020
| Region | Location by County | Growing Degree Days1 | ||
|---|---|---|---|---|
| 2020 | 2019 | 30-year Average | ||
| Augusta | St. Charles | 1088 | 1192 | 1196 |
| Hermann | Gasconade | 1027 | 1170 | 1153 |
| Ozark Highland | Phelps | 1122 | 1261 | 1252 |
| Ozark Mountain | Lawrence | 1121 | 1230 | 1230 |
| Southeast | Ste. Genevieve | 1106 | 1238 | 1263 |
| Central |
Boone | 1123 | 1189 | 1172 |
| Western | Ray | 1072 | 1075 | 1136 |
1 Growing degree days at base 50 from April 1 to June 20, 2020. Data compiled from Useful and Useable at https://mrcc.illinois.edu/U2U/gdd/. Click on link below to determine growing degree days in your area.
To determine the number of growing degree days accumulated in your area since April 1, use this tool.
Weather Outlook
Weather Outlook for Weekend
- Scattered thunderstorms
Week of June 29
- Temperatures upper 80s to mid 90s, which is above average historically
- Below normal precipitation forecast