A Survey of Viruses Found in Grapevine Cultivars Grown in Missouri
James Schoelz, Dean Volenberg and Maher Al Rawhanih
View the full Survey PDF
Contents
IntroductionMethodology used in the survey
Survey results
GRBV was prevalent in a majority of the cultivars sampled
Grapevine leafroll viruses were prevalent in a majority of the cultivars sampled
GVCV was detected almost exclusively in white-berried cultivars
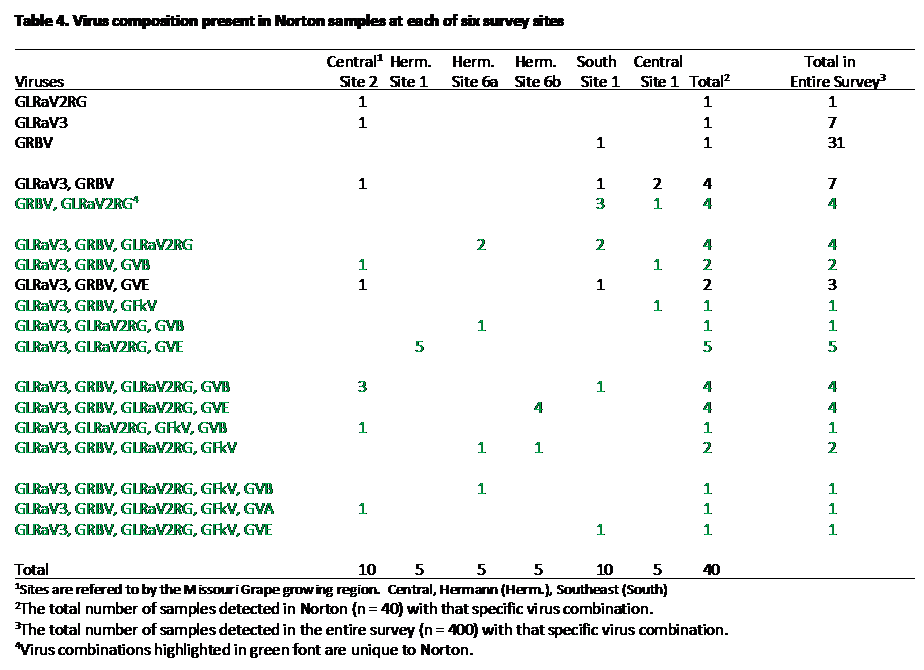
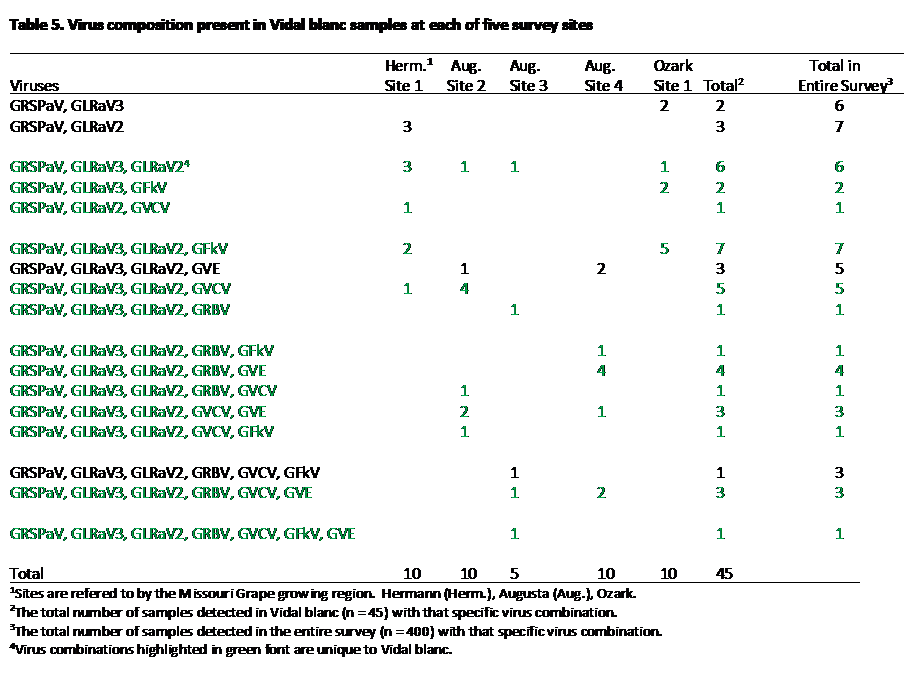
The most commonly grown cultivars in Missouri were typically infected with multiple viruses
Although not detected in the 2017 survey, the nematode-transmitted virus ToRSV was confirmed in 2018 surveys
Economically important viruses found in Missouri grapevines
Other viruses detected in the survey
Recommendations
References
Introduction
In 2017 the Grape and Wine Board supported a survey for grapevine viruses in Missouri vineyards. Previous surveys for grapevine viruses in Missouri were limited in scope and geography, focusing primarily on only a few locations in the state and on the viruses, Grapevine leafroll-associated virus 3 (GLRaV3), and several nepoviruses (i.e. Tomato ringspot virus (ToRSV), Tobacco Ringspot virus (TRSV) and Arabis mosaic virus (ArMV)), as well as Grapevine vein clearing virus (GVCV) (Guo et al., 2014; Lunden et al., 2010, Milkus, 2001; Milkus and Goodman, 1999; Zhang et al., 2011). In addition, Grapevine red blotch virus (GRBV) was found in one vineyard of Crimson Cabernet in 2016 (Schoelz et al., 2019). The survey in 2017 was valuable because it was the first comprehensive survey for viruses in Missouri vineyards. The survey in 2017 consisted of 400 samples collected from across the state from 25 different grapevine cultivars. Each sample was a composite of four leaves taken from each of four vines, collected randomly without regard for symptoms, and each sample was tested by Realtime-PCR at UC Davis for the presence of 27 different viruses. The 2017 survey revealed that a majority of the grape hybrids grown in Missouri were infected with at least one virus. Approximately 90% of the samples were infected with at least one virus, whereas 65% of the samples contained two or more viruses. The survey and the main results are described below.
Methodology used in the Survey
To obtain an accurate picture of the viruses that might be present in grapevines in Missouri, we utilized the same sampling pattern for every vineyard and collected samples without taking into account the presence of symptoms. The sampling pattern, which was in the shape of a “W”, is illustrated in Fig. 1 and briefly described here. The first sample was collected approximately three rows from the edge of the vineyard block and two thirds of the length of the row. At that location, we selected four leaves from each of four vines, two leaves from each cordon. Consequently, a single sample consisted of 16 leaves from four vines and was for the remainder of this report will be referred to as a composite sample. We typically collected 10 composite samples from a vineyard block, but in some blocks we only collected five composite samples (from sites 1, 4, 6, 8 and 10 in Fig. 1). In the 2017 survey, we collected 400 composite samples for a total of 1600 vines samples for viruses.
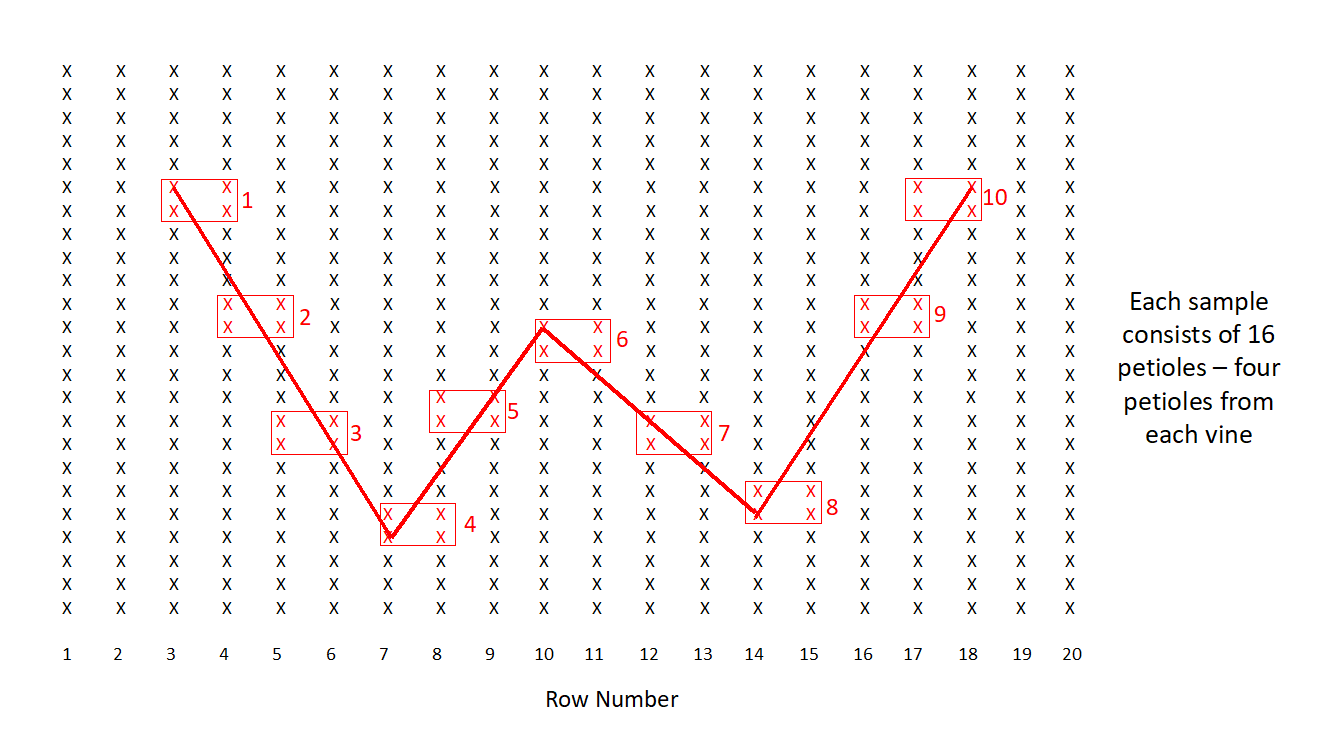
Figure 1. Sampling pattern used for the collection of 10 samples from a vineyard block. The x’s illustrated in red illustrate the location of the four vines that comprise a composite sample. Four leaves were collected from each vine.
Composite samples were collected from vineyards beginning the last week of June and continued sampling through July of 2017. Once collected, the leaves were returned to MU, where slices were taken from each of the petioles in a composite sample for a total fresh weight of 80 mg per composite sample. One complete set of the 400 composite samples was shipped to Foundation Plant Services (FPS) at UC Davis for further processing and a second complete set was stored in a -80 freezer located in Waters Hall at MU. At FPS, the total nucleic acids (RNA and DNA) were isolated from each of the plant samples and then assayed for 27 different viruses by quantitative Realtime PCR. The viruses included in the survey are listed in Table 1.
In addition to testing for viruses, the soil at the base of the vine samples was analyzed for the presence of the nematode Xiphinema and Longidorus, two genera capable of vectoring grapevine viruses, Grapevine fanleaf virus, TRSV, ToRSV, and ArMV. Soil was collected from the base of each of the grapevines that were chosen for leaf samples. The soil from each vineyard was then combined into a single sample and analyzed for the presence of nematodes by SCN Diagnostics, the Plant and Nematode Screening Service at MU.
Table 1. Grapevine viruses included in the 2017 test.1
Nepoviruses:
Grapevine fanleaf virus (GFLV)
Tomato ringspot virus (ToRSV)
Arabis mosaic virus (ArMV)
Tobacco ringspot virus (TRSV)
DNA viruses:
Grapevine red blotch virus (GRBV)
Grapevine vein clearing virus (GVCV)
Trichovirus:
Grapevine Pinot gris virus (GPGV)
Fovevirus:
Grapevine Rupestris stem pitting virus (GRSPaV)
Maculavirus:
Grapevine fleck virus (GFkV)
Vitiviruses:
Grapevine virus A (GVA)
Grapevine virus B (GVB)
Grapevine virus D (GVD)
Grapevine virus E (GVE)
Grapevine virus F (GVF)
Closteroviruses:
Grapevine leafroll-associated virus 1 (GLRaV-1)
Grapevine leafroll-associated virus 2 (GLRaV-2)
Grapevine leafroll-associated virus 2RG (GLRaV-2RG)
Grapevine leafroll-associated virus 3 (GLRaV-3)
Grapevine leafroll-associated virus 3e (GLRaV-3e)
Grapevine leafroll-associated virus 4 (GLRaV-4)
Grapevine leafroll-associated virus 5 (GLRaV-5)
Grapevine leafroll-associated virus 6 (GLRaV-6)
Grapevine leafroll-associated virus 7 (GLRaV-7)
Grapevine leafroll-associated virus 8 (GLRaV-8)
Grapevine leafroll-associated virus 9 (GLRaV-9)
Grapevine leafroll-associated virus 10 (GLRaV-10)
Grapevine leafroll-associated virus Car (GLRaVCar)
1 Total nucleic acid samples were extracted from petioles and analyzed using reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) to detect viral RNA/DNA.
2 Viruses in bold were detected in the survey.
Survey results
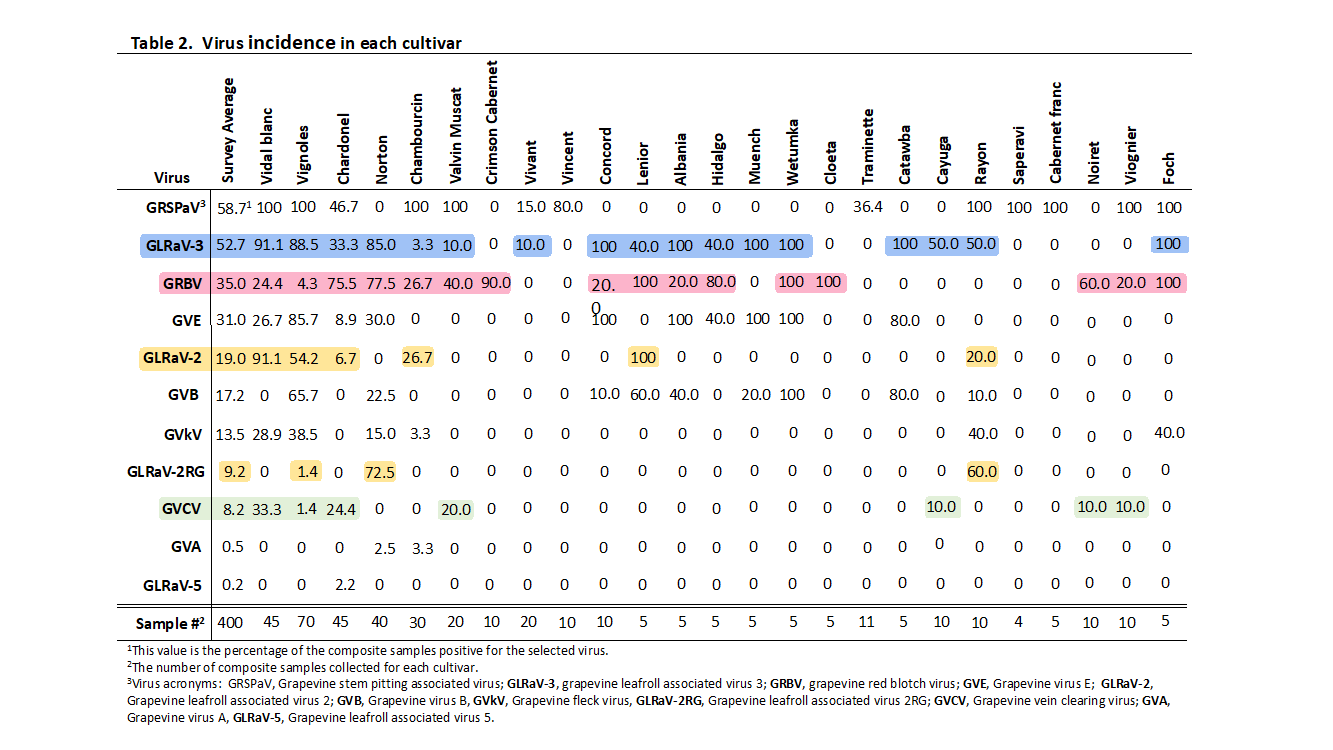
A total of 25 grape cultivars were sampled, and the results for each cultivar are summarized in Table 2. The number of composite samples taken for each cultivar is listed at the bottom of the table (e.g., there were 45 composite samples collected for Vignoles) and the values listed for each virus/cultivar combination is the percentage of the composite samples positive for that virus. The top three most prevalent viruses in grapevine cultivars were Grapevine stem pitting associated virus (GRSPaV), GLRaV-3, and GRBV, with incidences of 58.7%, 52.7% and 35.0%, respectively (Table 2). However, a discussion of the average incidence is deceptive. For example, 58.7% of the samples contained GRSPaV, but 100% of the Vidal blanc samples contained this virus, whereas this virus was completely absent from Norton (Table 2). Consequently, it is better to look at the virus composition in individual cultivars rather than the average results from the survey. The major findings of the survey are summarized below.
GRBV was prevalent in a majority of the cultivars sampled in the 2017 survey. GRBV was first discovered in Missouri in 2016 in the cultivar ‘Crimson Cabernet’ (Schoelz et al., 2019). The 2017 survey revealed the widespread occurence of GRBV, as it was detected in 17 of the 25 grape cultivars tested (Table 2, GRBV samples highlighted in blue). GRBV was detected in 77% of the 40 Norton samples collected from six vineyards in the 2017 survey (Table 2). Significantly, we have never observed the typical red leaf symptoms associated with GRBV infection in Norton, and we hypothesize that Norton leaves may remain asymptomatic during the growing season. It is unknown if GRBV has any effect on berry quality. GRBV was detected in 26.7% of the 30 Chambourcin samples, which were collected from five vineyards around the state (Table 2). Of the seven cultivars most widely grown in Missouri (USDA Natl. Ag Statistics Service Missouri, 2016), GRBV was detected in approximately 75% of Chardonel and Norton samples, and was present in approximately 20-25% of Vidal blanc, Chambourcin, and Concord. Vignoles was relatively free of this virus (4.3% of samples positive), and GRBV was absent in Catawba samples (Table 2).
Current management recommendations developed for GRBV-infected vineyards in California are drastic, as vineyards of V. vinifera with 30% GRBV infection are suggested to be removed entirely (Ricketts et al., 2016). In the 2017 survey, GRBV was detected in ten cultivars at a level above 30%. Since all samples collected in 2017 were composites of four vines, the infection rate in a sample could actually vary from 25% to 100%. To evaluate the true incidence of GRBV in Missouri, we followed up in 2018 with testing of individual vines of Norton, Chambourcin, and Crimson Cabernet at several locations. In general, the testing of individual vines in 2018 confirmed the results obtained with the composite samples in 2017. For example, GRBV was detected in 90% of the 10 Crimson Cabernet composite samples collected from two locations in 2017, and was detected at similar rates in the follow-up survey in 2018.
Prior to 2017, the only cultivar in Missouri that appeared to have any evidence of GRBV symptoms was Crimson Cabernet. Symptom expression in Crimson Cabernet infected with GRBV can anecdotally be attributed to genetic parentage, as Crimson Cabernet is 62.5% V. vinifera and 37.5% V. aestivalis. To determine whether GRBV symptoms could be observed in Norton and Chambourcin leaves, individual leaves were collected at the end of the growing season in 2018, photographed and then scored for the presence or absence of the GRBV virus by PCR. It is important to note that symptoms of GRBV infection can be confused with symptoms for GLRaV-3. Significantly, the site chosen for sampling Chambourcin leaves had been found in the 2017 survey to be free of GLRaV-3, so we could assess the contribution of GRBV by itself to symptomatology in infected vines. This was not the case for Norton, as the 2017 survey showed that 85% of Norton samples were infected with GLRaV-3.
Figure 2 illustrates the Chambourcin leaves sampled for GRBV. Our preliminary results suggest that GRBV infection in Chambourcin is associated with a bright red symptom in the interveinal tissue, with the veins remaining green. This is best exemplified in leaf sample 48, but is also clearly observed in leaves 42, 43, and 47 (Fig. 2). This standard is not perfect, as some leaves may be asymptomatic (Fig. 2, e.g. leaf 52). Nonetheless, a visual marker indicative of GRBV infection would be valuable, even if there are some false negatives. By contrast, Chambourcin leaves negative for GRBV may develop a generalized bronzing that extends over the entire leaf or may remain green (Fig. 2). In the case of Norton, there was no difference in appearance between the GRBV-positive and GRBV-negative leaves (Fig. 3), indicating that at the level of symptomatology, Norton appears tolerant to infection of both GRBV and GLRaV-3. However, although Norton leaves infected with these viruses are asymptomatic, there still could be significant effects on host physiology.
Grapevine leafroll viruses were prevalent in a majority of the cultivars sampled in the 2017 survey. Grapevine leafroll disease (GLD) is considered the most important virus disease of grapevines worldwide (Maree et al., 2013) and it is caused by a complex of several distinct viruses (Martelli et al., 2012; Sharma et al., 2011). Two distinct leafroll viruses (GLRaV-3, GLRaV-2/GLRaV-2RG) were detected at significant levels in the 2017 survey, whereas a third virus (GLRaV-5) was found in only one of the 400 composite samples (Table 2, GLRaV-3 is highlighted in blue whereas GLRaV-2/GLRaV-2RG are highlighted in gold). Of these three viruses, GLRaV-3 is considered the most important causal agent of GLD (Maree et al., 2013). In the 2017 survey for GLRaV-3 in Missouri, GLRaV-3 was found in 19 of the 25 grape hybrid cultivars surveyed and overall was detected in 52% of all samples (Table 2). For the grape cultivars sampled at five or more locations, GLRaV-3 was detected at a very high level (over 85%) in Vidal blanc, Vignoles, and Norton. GLRaV-3 was also detected at high levels in Concord, Muench, Wetumpka, Catawba and Marechal Foch (Table 2). However, for these cultivars the sample size was small and typically came from only one location, so it is not possible to state whether the high levels of infection are indicative of GLRaV-3 levels across the state for those cultivars. Significantly, we have never observed the typical red leaf symptoms associated with GLRaV-3 infection in Norton, and we hypothesize that Norton leaves may remain asymptomatic during the growing season. It is unknown if GLRaV-3 has any effect on berry quality of Norton, although it has been shown to alter berry quality of other grape hybrids (Kovacs et al., 2011).
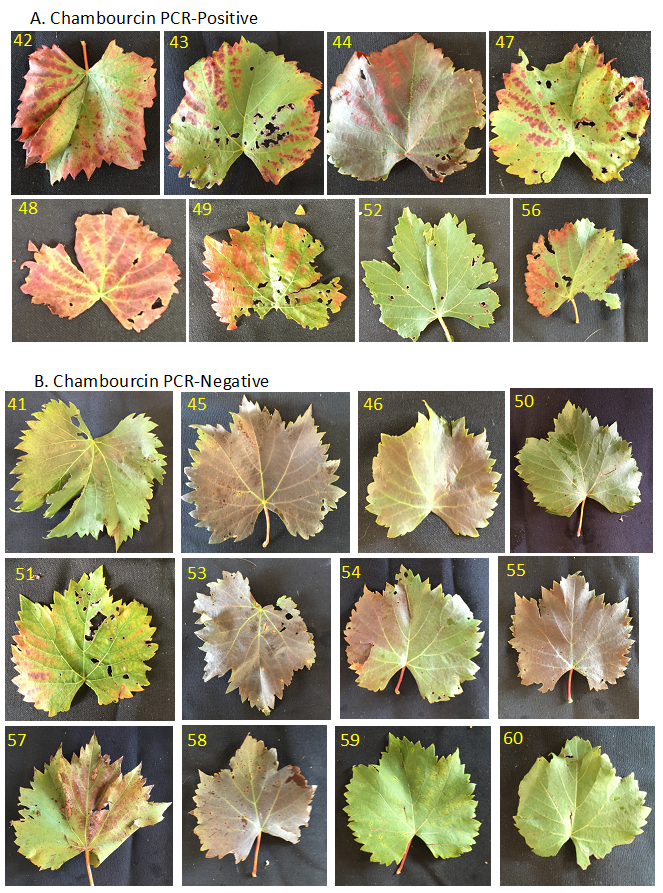
Figure 2. Association of Chambourcin leaf symptoms with GRBV infection.
A. Chambourcin samples that were PCR positive for GRBV.
B. Chambourcin samples that were PCR negative for GRBV.The yellow number in the top left corner of each image indicates the sample number.

Figure 3. Association of Norton leaf symptoms with GRBV infection.
A. Norton samples that were PCR positive for GRBV.
B. Norton samples that were PCR negative for GRBV. The yellow number in the top left corner of each image indicates the sample number.
In the 2017 statewide survey for viruses in Missouri, GLRaV-2 was found in 7 and GLRaV-2RG was found in four of the 25 grape hybrid cultivars we surveyed (Table 2). Although these viruses were not as widely detected as GLRaV-3, there were some cultivars in which the incidence was high. For example, GLRaV-2RG was detected in 72.5% of the 40 Norton samples collected from six vineyards (Table 2), whereas GLRaV-2 was found in 91.1% of Vidal blanc, 54.2% of Vignoles, and 26.7% of Chambourcin samples.
GVCV was detected almost exclusively in white-berried cultivars. GVCV, a grapevine virus that was first discovered and characterized in Missouri (Guo et al., 2014; Lunden et al., 2010, Zhang et al., 2011), was detected in 8.2% of the composite samples taken during the 2017 survey (Table 2, text highlighted in green). The low survey incidence of GVCV in the survey was likely skewed by the observation that GVCV was absent from all red-berried cultivars, with the exception of Noiret. In fact, GVCV was detected at moderate levels in Vidal blanc, Chardonel, and Valvin Muscat (Table 2., 33.3%, 24.4% and 20.0%, respectively). Consequently, GVCV might be considered a significant problem for specific white grape cultivars, but does not appear to be an issue for red grape cultivars. Recently, it was shown that Chambourcin is resistant to GVCV (Guo et al., 2014); it may be that resistance is common in other red grape cultivars.
The most commonly grown cultivars in Missouri were typically infected with multiple viruses. The 2017 survey found that 90.2% of the composite samples contained at least one virus. Two or more viruses were detected in approximately 65% of the samples, with an upper limit of seven viruses detected in one sample. Interestingly, an analysis of the survey results indicated that grapevine cultivars appear to be infected by specific virus combinations that are for the most part, unique to that cultivar. This is most clearly seen in three of the cultivars most commonly grown in Missouri, the cultivars Vignoles, Norton, and Vidal Blanc.
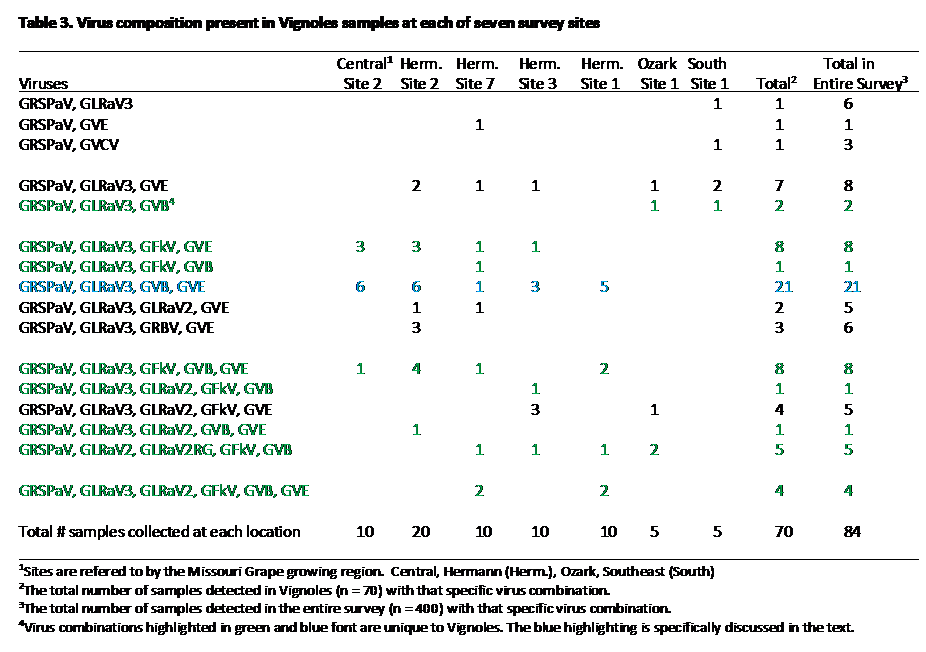
For example, every one of the 70 composite samples collected for Vignoles contained two or more viruses (Table 3). GRSPaV was present in every sample, whereas GLRaV-3 was present in 90%. By contrast, GRBV and GVCV were nearly absent from this cultivar, as they were found in 4.2% and 1.4% of the composite samples, respectively. One of the strengths of the survey is that multiple Vignoles vineyards were surveyed, revealing that virus combinations discovered in Vignoles were largely unique to Vignoles. For example, composite samples containing GRSPaV, GLRaV3, GVB, and GVE (Table 3., highlighted with blue lettering) were found at five different vineyards, and this combination occurred only with Vignoles. This pattern was observed throughout; in Table 3, virus combinations unique to Vignoles (i.e not found with other cultivars) are highlighted with green lettering. What this suggests is that virus infections in Vignoles have more in common with other Vignoles vineyards across the state, rather than with virus infections of other cultivars planted adjacent to Vignoles. This in turn implies that the viruses infecting Vignoles are more likely to have arisen through infected planting material than through spread by a vector.
The infection pattern for Norton was distinctly different from Vignoles (Table 4). In contrast to Vignoles, GRSPaV and GLRaV-2 were absent from all samples. Furthermore, GRBV and GLRaV-2RG were detected in 77.5% and 72.5% of the composite samples, respectively. These two viruses that were nearly absent from Vignoles samples. The only virus that was detected at a high levels in both Norton (at 85.0%) and Vignoles was GLRaV-3 (at 88.5%). In Table 4, virus combinations unique to Norton (i.e not found with other cultivars) are highlighted with green lettering. All of the Norton composite samples contained at least one virus, and 92.5% carried two more viruses; of samples that contained more than one virus, all contained some combination of GRBV, GLRaV-3 and GLRaV-2RG; 42.5% of the Norton samples contained all three viruses (GRBV, GLRaV-3, and GLRaV-2RG), whereas another 50% of the samples consisted of one of three combinations: GRBV and GLRaV-2RG, GLRaV-3 and GLRaV2RG, or GLRaV-3 and GRBV (Table 4).
The infection pattern for Vidal blanc was mixture of elements from both Vignoles and Norton (Table 5). For example, GRSPaV was detected in 100% of the Vidal Blanc samples, similar to Vignoles, but also a significant percentage (24%) also carried GRBV. In addition, 91.1% of the samples carried GLRaV-3. One characteristic that distinguished Vidal blanc from both Norton and Chambourcin was that one third of the samples contained GVCV. In addition, Vidal blanc had one of the highest infection rates for GLRaV-2 (at 91.1%). As with Vignoles and Norton, most of the virus combinations detected in Vidal blanc were unique to that variety (Table 5, highlighted with green text).
It is important to note that, in a process called synergism, the severity of disease caused by two or more unrelated viruses in the same plant can be much stronger than what would be expected by the sum of the disease effects in plants infected with individual viruses (Hull at al. 2002). Most studies have focused on the harmful effects of GRBV and GLRaV-3 in isolation. However, given the multiple viruses detected in the composite samples, it will be important to examine specific combinations of viruses detected in the cultivars grown in Missouri.
Although not detected in the 2017 survey, the nematode-transmitted virus ToRSV was confirmed in 2018 surveys. One unexpected result of the 2017 survey was that no nematode transmitted viruses were detected, even though GFLV, ToRSV, TRSV, and ARMV were included in the test. One reason why this result was surprising was that an earlier survey of five Missouri vineyards found the presence of ToRSV and ArMV (Milkus, 2001). Furthermore, the presence of ToRSV was confirmed by ELISA in one Vidal blanc vineyard in Augusta in 2016, even though an RT-PCR assay failed to detect the virus in the same vineyard in 2017. Consequently, one of the goals of a follow up survey in 2018 was to reconcile the different assays the disparate results obtained in 2016 and 2017 surveys.

Figure 4. Poor berry set in Vignole vine infected with ToRSV.
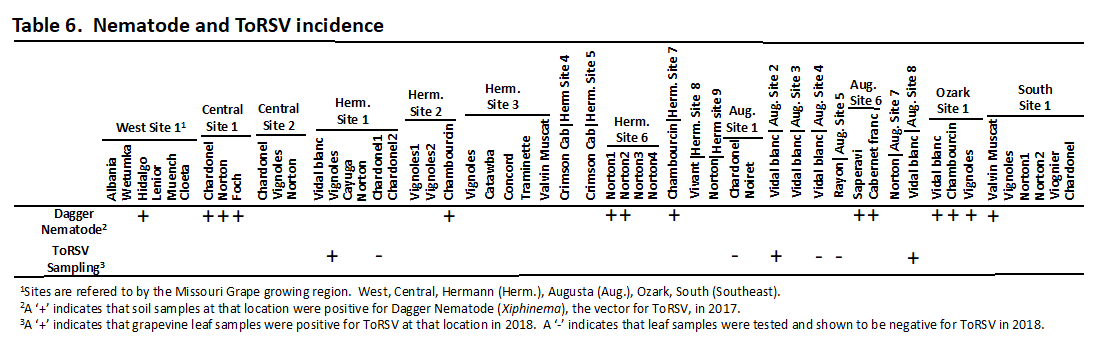
ToRSV typically induces poor berry set and size in berry clusters (Fig. 4) (Rowhani et al., 2017), so in 2018 we surveyed seven vineyards (Three Vidal blanc, two Chardonel, one Vignoles, and one Rayon) for grape clusters that exhibited symptoms of ToRSV infection. ToRSV symptoms were found at two Vidal blanc and one Vignoles vineyards (Table 6). Samples from each vineyard were subsequently tested for ToRSV using the same ELISA test used in the 2016 survey (an antibody-based test that detects one of the proteins of ToRSV) and the RT-PCR test from 2017 (a test that is based on the ToRSV RNA). In the 2018 survey, the ELISA test confirmed the presence of ToRSV, whereas the RT-PCR failed to detect ToRSV. We noted that the RT-PCR test targeted a different portion of ToRSV than the ELISA test. Consequently, we developed new RT-PCR primers that corresponded more closely to the same ToRSV target as was used for the ELISA test, and in this case our new RT-PCR assay successfully detected ToRSV from samples collected at all three locations (Table 6). A nucleotide sequence of the PCR products generated in our new RT-PCR assay confirmed that we had amplified ToRSV and that the virus was related to other ToRSV strains recovered from grapevines in California, Illinois, New York, and Canada (Li et al., 2011; Yao et al., 2017).
We concluded that we failed to detect ToRSV in the 2017 survey because the ToRSV strains in Missouri significantly differed from the strain that was used for development of the RT-PCR assay. However, we were able to redesign the RT-PCR assay so that it now could be used for detection of ToRSV, and both the ELISA and the new RT-PCR confirm the presence of ToRSV in grape cultivars grown in Missouri.
In the 2017 survey, the soil at each vineyard was sampled for the presence of nematodes responsible for the transmission of the viruses. Xiphimema, the genus responsible for transmission of ToRSV, was found at approximately one third of the vineyards sampled (Table 6). Interestingly, Xiphimema was not found at the two locations in which vines infected with ToRSV were detected. This is encouraging, because ToRSV will not spread from one vine to another in the absence of the nematode vector. The caveat for this observation is that only a small portion of the vineyard soil can be sampled for the presence of nematodes.
Consequently, there is a possibility that the limited soil samples taken for the nematode analysis were insufficient in number. It will be important to continue to monitor for Xiphimema in vineyards in which ToRSV infections of vines have been confirmed.
Economically Important Viruses found in Missouri grapevines
Grapevine leafroll-associated virus 3 (GLRaV-3)
GLRaV-3 is considered the primary causal agent of GLD, the most important virus disease of grapevines (Maree et al., 2013). Symptoms of GLD are most prominent in red-berried cultivars of V. vinifera, as symptomatic leaves accumulate anthocyanin pigments due to induction of the anthocyanin biosynthetic pathway (Gutha et al., 2010), which causes the leaves to turn reddish-purple. Typically, a narrow band of tissue along the major veins remains green (Burger et al., 2017; Naidu et al., 2014) in symptomatic leaves. By contrast, white-berried cultivars may show mild yellowing. Both types of cultivars may also exhibit a downward curling of the veins. Symptoms may begin in the bottom of the canopy as early as véraison, but symptom development is dependent on a number of factors, including the cultivar, the choice of scion and rootstock, and the environment. In contrast to V. vinifera, symptoms of GLD have not been observed in American grape species and in hybrids, even though there is no evidence of resistance in these Vitis species.
The effect of GLD on vine physiology has been studied extensively, as summarized in Reynolds, (2017). In V. vinifera, GLD has been shown to affect vine health in a number of ways, including a reduction in photosynthesis, reductions in cluster number, cluster weight, berry weight, and vine size. GLD may also affect wine quality through delays in fruit maturity, as well as through alterations in soluble solids, titratable acids (TA), total phenolics and total tannins (Burger et al., 2017; Naidu et al., 2014). Based on studies on V. vinifera grown on the West Coast and New York, the potential economic impact could be significant. One study conducted in the Finger Lakes region of New York estimated losses associated with GLRaV-3 at $25,000-40,000 per hectare in Cabernet Franc vineyards (Atallah et al., 2012), with comparable estimated losses for Cabernet Sauvignon and Merlot vineyards in California and Washington (Naidu and Walsh, 2015; Ricketts et al., 2015).
Although much has been published on the effect of GLRaV-3 on the vine health of V. vinifera, there currently is a gap in our knowledge concerning the impact of this virus on the types of grape hybrids grown in Missouri. In fact, only one study has examined the influence of virus infections on hybrid grapes. Kovacs et al., 2001 examined the impact of GLRaV-3 on Vidal blanc and St. Vincent cultivars. They showed that GLRaV-3 caused a reduction in berry weight and an increase in titratable acid levels.
Grapevine red blotch virus (GRBV)
GRBV was only recently recognized as a distinct virus pathogen of grapes in 2012 (Al Rwahnih et al., 2013: Krenz et al., 2012), but it is now recognized as an important disease throughout the U.S. (Sudarshana et al., 2015), including Missouri (Schoelz et al., 2019). It is considered that GRBV had been causing disease for many years before this in the U.S. but the symptoms were confused with those caused by GLRaV-3. The economic impact of GRBV is comparable to GLRaV-3. Perhaps because of its relative newness as a distinct virus disease, very little is known about how GRBV might affect grapes. GRBV induces red symptoms in leaves of red-berried V. vinifera cultivars, GRBV typically beginning in older leaves late in the season (Cieniewicz et al., 2017). Leaves with strong symptoms may prematurely drop off the vine. In white berried cultivars leaves may develop chlorosis that turn necrotic as the season progresses. GRBV also is known to delay fruit ripening, altering total soluble solids, decreasing Brix in fruit and anthocyanin contents in berry skin (Blanco-Ulate et al. 2017; Cieniewicz et al., 2017). Symptoms in red-berried cultivars caused by GRBV may be confused with GLRaV-3, but also could be similar to abiotic stresses that include magnesium or potassium nutrient deficiencies. Ricketts and coworkers (2016) estimated that the economic impact of GRBV ranged “from $22,213-$68,548 per hectare over a 25-year productive life span of Cabernet Sauvignon and Merlot vineyards, depending on the level of initial infection and price penalty for suboptimal fruit quality”. Currently we do not know if brix levels stall out in Norton or Chambourcin GRBV infected vineyards in Missouri. However, there is some anecdotal evidence that at least in the case of Norton that this does not occur. Duncan (2017) examined the berry quality of three different Norton vineyards in Missouri and one of these vineyards was identified as being GRBV infected by Schoelz et al. (2019). This vineyard had similar fruit quality (Brix and anthocyanins) as the other two vineyards that have a very low incidence of GRBV.
Grapevine leafroll-associated virus 2 (GLRaV-2) and the variant GLRaV-2RG
GLRaV-2 has a long history of association with GLD, but any symptoms attributed to this virus have been complicated by the observation that it is frequently found with other viruses (Angelini et al., 2017). It is generally accepted that strains of GLRaV-2 may cause two distinct diseases; leafroll and graft incompatibility. However, the induction of symptoms by strains of this virus has been described in the literature as weak or erratic, and it may be specific to certain host/virus combinations. All V. vinifera cultivars and hybrids are susceptible to GLRaV-2, and the virus has also recently been found in wild Vitis species native to North America (Abu Ghanem-Sabanadzovic and Sabanadzovic, 2015; Klaassen et al., 2011). GLRaV-2RG is considered a variant of GLRaV-2 and is thought to cause a severe decline of cv. ‘Redglobe” grafted onto several rootstocks (Uyemoto and Rowhani, 2001). We have included GLRaV-2RG and GLRaV-2 in this section because they were frequently found in association with GRBV in either Norton or Chambourcin samples collected in our survey in 2017. Consequently, these viruses might contribute to declines in vine and/or berry health through a synergistic effect with GRBV or GLRaV-3.
Grapevine vein clearing virus (GVCV)
GVCV was first discovered in Missouri (Zhang et al., 2011) and appears to be confined the Midwest United States (Guo et al., 2014). The virus has been associated with a decline in vine health (Lunden et al., 2010; Guo et al., 2014), and it remains to be seen if it has any effect on berry quality. The 2017 survey suggested that the GVCV is largely a problem in white wine cultivars; it was detected in approximately one third of the Vidal blanc samples, one fourth of the Chardonel samples and one fifth of the Valvin muscat samples, but was only found in 1-2% of Vignoles. Consequently, this virus may cause serious problem for some grape cultivars, but is may not be an issue for many other cultivars.
Other viruses detected in the survey
Several other grapevine viruses were discovered in a significant number of samples including GRSPaV (58.7%), the vitiviruses (GVE- 31%, GVB-17.2%, GVA-0.5%) and GFkV (13.5%). However, it is unclear how much they affect production (Meng and Rowhani, 2017; Minafra et al., 2017; Sabanadzovic et al., 2017) For example, Meng and Rowhani (2017) state “It is commonly believed in the grapevine virology community that GRSPaV is generally a benign virus, causing asymptomatic or mild infections.” Similar statements describe infections of grapes with the vitiviruses and GFkV. Meng and Rowhani (2017) acknowledge that more research should be done to examine the true effect of GRSPaV on vine health, but they also note that GRSPaV has been “one of the most recalcitrant viruses to eliminate through conventional means such as heat therapy and shoot tip culture.” Given the near ubiquity of GRSPaV in many of the grape cultivars grown in Missouri, the best strategy would be to focus on viruses that have been documented to be detrimental to vine health and grape quality.
Recommendations
- 1. One of the surprising results of this survey was the widespread incidence of GRBV and GLRaV-3 in cultivars grown in Missouri. Although the detrimental effects of these viruses have been well-documented for V. vinifera, it is unclear what effect they might have on the physiology of grape hybrids. Further research should be directed towards understanding their effect on vine health and berry quality of the grape hybrids grown in Missouri.
- 2. In addition to examining the individual impact of GRBV and GLRaV-3 on grape hybrids, it is important to identify grapevines infected with multiple viruses and determine the impact that these specific virus combinations may have on the health of the cultivars grown in Missouri.
- 3. At this point, it is too early to tell what effect viruses such as GLRaV-3 and GRBV have on vine health and berry quality of the cultivars grown in Missouri. However, if the experience gained from the wine industry on the west coast and New York is any indication, it would be prudent to develop a robust system for testing cultivars for the major viruses. In the future, this system could be important in the identification and gradual replacement of vines with the most detrimental combinations of viruses.
- 4. At this point, it is unclear whether the cultivars infected with either GRBV or GLRaV3 develop any obvious symptoms of infection. Preliminary evidence suggests that Chambourcin infected with GRBV may develop symptoms late in the season, but this needs to be confirmed. Efforts should be made to confirm potential symptoms in Chambourcin and determine if symptoms are apparent in other varieties. An ELISA or PCR-based test would still be necessary to confirm infection, but an initial screen based on symptoms would be a cost-effective method to focus the sampling process to identify virus infections before the molecular assay.
- 5. In contrast to GRBV and GLRaV-3, ToRSV induces prominent symptoms in infected vines and it clearly has a detrimental effect on berry set and size in berry clusters (Fig. 4). Growers should be educated on the symptoms of this virus disease and encouraged to submit samples for testing. At present, the most reliable and economical test for ToRSV in our hands is the ELISA. Once a vine is confirmed to be infected with ToRSV, it should be removed and replaced with a virus-free vine. Furthermore, the soil surrounding the vine should be tested for the presence of the nematode vector.
References
- Abu Ghanem-Sabanadzovic N, Sabanadzovic S. 2015. First report of Grapevine leafroll-associated virus 2 infected muscadine (Vitis rotundifolia) and summer grape (Vitis aestivalis) in the United States. Plant Disease 99, 1.
- Al Rwahnih M, Ashita MD, Anderson M, Rowhani A, Uyemoto JK, Sudarshana MR. 2013. Association of a DNA virus with grapevines affected by red blotch disease in California. Phytopathology 103, 1069-1076.
- Angelini E, Aboughanem-Sabanadzovic N, Dolja VV, Meng B. 2017. Grapevine leafroll-associated virus 2. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Atallah SS, Gómez MI, Fuchs MF, Martinson TE. 2012. Economic impact of grapevine leafroll disease on Vitis vinifera cv. Cabernet franc in Finger Lakes vineyards of New York. American Journal of Enology and Viticulture 63, 73-79.
- Blanco-Ulate B., Hopfer H, Figueroa-Balderas R, Ye Z, Rivero RM, Albacete A, Pérez-Alfocea F, Koyama R, Anderson MM, Smith RJ, Ebeler SE, Cantu D. 2017. Red blotch disease alters grape berry development and metabolism by interfering with transcriptional and hormonal regulation of ripening. J. Exp. Botany 68:1225-1238.
- Burger JT, Maree HJ, Gouveia P, Naidu RA. 2017. Grapevine leafroll-associated virus 3. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Cieniewicz E, Perry K, Fuchs M. 2017. Grapevine red blotch: Molecular biology of the virus and management of the disease. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Duncan C. 2017. Characterization of berry ripening in Missouri Norton wine grapes. Thesis, University of Missouri-Columbia. Pp 39-44.
- Guo Q., Honesty S., Xu ML, Zhang Y, Schoelz J, Qiu W. 2014. Genetic diversity and tissue and host specificity of Grapevine vein clearing virus. Phytopathology 104, 539-547.
- Gutha LR, Casassa LF, Harbertson JF, Naidu RA. 2010. Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera) leaves. BMC Plant Biology 10, 187.
- Hull R. 2002. Matthews’ Plant Virology, Fourth edition, Academic Press, San Diego, 1001 pgs.
- Klaassen VA, Sim ST, Dangl GS, Osman F, Al Rwahnih M, Rowhani A, Golino DA. 2011. Vitis californica and Vitis californica x Vitis vinifera hybrids are hosts for Grapevine leafroll-associated virus 2 and -3 and Grapevine virus A and B. Plant Disease 65, 657-665.
- Kovacs LG, Hanami H, Fortenberry M, Kaps ML. 2001. Latent infection by leafroll agent GLRaV-3 is linked to lower fruit quality in French-American hybrid grapevines Vidal blanc and St. Vincent. American Journal of Enology and Viticulture 52, 254-259.
- Krenz B, Thompson JR, McLane HL, Fuchs M, Perry KL. 2014. Grapevine red blotch-associated virus is widespread in the United States. Phytopathology 104, 1232-1240.
- Li R, Mock R, Fuchs M, Halbrendt J, Howell B, Liu Z. 2011. Characterization of the partial RNA1 and RNA2 3’ untranslated region of Tomato ringspot virus isolates from North America. Can. J. Plant Pathol. 33, 94-99.
- Lunden S, Meng B, Avery Jr J, Qiu W. 2010. Association of Grapevine fanleaf virus, Tomato ringspot virus, and Grapevine rupestris stem pitting-associated virus with at grapevine vein-clearing complex on var. Chardonnay. Eur. J. Plant Pathol. 126, 135-144.
- Maree HJ, Almeida RP, Bester R, Chooi K, Cohen D, Dolja VV, Fuchs MF, Golino DA, Jooste AE, Martelli GP, Naidu RA, Rowhani A, Saldarelli P, Burger J. 2013. Grapevine leafroll-associated virus 3. Frontiers in Microbiology 4, 82.
- Martelli GP, Abou Ghanem-Sabanadzovic AA, Agranowsky M, Al Rawhanih M, Dolja VV, Dovas CI, Fuchs M, Gugerli P, Hu JS, Jelkmann W, Katis N, Maliogka VI, Melzer MJ, Menzel W, Minafra A, Rott ME, Rowhani A, Sabanadzovic S, Saldarelli P. 2012. Taxonomic revision of the family Closteroviridae with special reference to the grapevine leafroll-associated member of the genus Ampelovirus and the putative species unassigned to the family, Journal of Plant Pathology 94, 7-19.
- Meng B, Rowhani A. 2017. Grapevine rupestris stem pitting-associated virus. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Milkus BN. 2001. Incidence of four NEPO viruses in Missouri vineyards. Am. J. Enol. Vitic. 52, 56-57.
- Milkus BN, Goodman RN. 1999. A survey of Missouri Vineyards for the presence of five grape viruses. Am. J. Enol. Vitic. 50:133-134.
- Minafra A, Mawassi M, Goszcynski D, Saldarelli P. 2017. Grapevine vitiviruses. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Naidu RA, Rowhani A, Fuchs M, Golino D, Martelli GP. 2014. Grapevine leafroll: a complex viral disease affecting a high-value fruit crop. Plant Disease 98, 1172-1185.
- Naidu RA, Walsh D. 2015. Is ‘grape virus tax’ hitting your pocketbook? Good Fruit Grower 66, 10-11.
- Reynolds AG. 2017. The Grapevine, viticulture, and winemaking: a brief introduction. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Ricketts KD, Gomez MJ, Atallah SS, Fuchs MF, Martinson TE, Battany MC, Bettiga LJ, Cooper ML, Verdegaal PS, Smith RJ. 2015. Reducing the economic impact of grapevine leafroll disease in California: identifying optimal disease management strategies. American Journal of Enology and Viticulture 66, 138-147.
- Ricketts KD, Gomez MI, Fuchs MF, Martinson TE, Smith RJ, Cooper ML, Moyer M, Wise A. 2016. Mitigating the economic impact of grapevine red blotch: optimizing disease management strategies in U.S. vineyards. American Journal of Enology and Viticulture 68, 127-135.
- Rowhani A, Daubert SD, Uyemoto JK, Al Rwahnih M. 2017. American Nepoviruses. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Sabanadzovic S, Aboughanem-Sabanadzovic N, Martelli GP. 2017. Grapevine fleck and similar viruses. In Grapevine Viruses: Molecular Biology, diagnostics and Management (Meng B, Martelli GP, Golino DA, Fuchs M, eds) 698 pgs.
- Schoelz JE, Adhab M, Qiu W, Peterson S, Volenberg D. 2019. First report of red blotch virus in hybrid grapes in Missouri. Plant Disease 103, 379.
- Sharma AM, Wang J, Duffy S, Zhang MK, Wong A, Rashed ML, Cooper KM, Daane KM, Almeida RPP. 2011. Occurrence of grapevine leafroll-associated virus complex in Napa valley. PLoS One 6:10.
- Sudarshana MR, Perry KL, Fuchs MF. 2015. Grapevine red blotch-associated virus, an emerging threat to the grapevine industry. Phytopathology 105, 1026-1032.
- USDA National Agricultural Statistics Service Missouri. 2016. Missouri Grape Facts. https://missouriwine.org/sites/default/files/2016%20Grape%20Facts_0.pdf
- Uyemoto JK, Rowhani A, Luvisis D, Krag R. 2001. New closterovirus in ‘Redglobe’ grape causes decline of grafted plants. California Agriculture 55, 28-31.
- Yao X-L, Han J, Domier LL, Qu F, Lewis Ivey ML. 2017. First report of Tomato ringspot in an Ohio Vineyard. Plant Disease 102, 259.
- Zhang Y, Singh K, Kaur R, Qiu W. 2011. Association of a novel DNA virus with the grapevine vein-clearing and vine decline syndrome. Phytopathology 101, 1081-1090.